Corin gets FDA approval of Unity Knee insert for robotic knee replacement
Robotics Business Review
OCTOBER 6, 2024
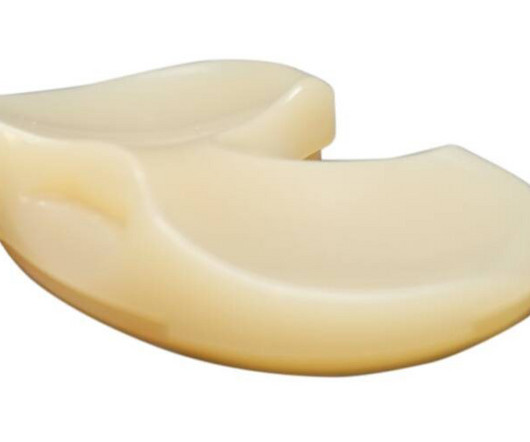
The Unity Knee MC tibial insert. Source: Corin Corin Group PLC last week said it has received 401(k) clearance from the U.S. Food and Drug Administration for for its Unity Knee medial constrained, or MC, tibial insert. It is suitable for use with or without the posterior cruciate ligament (PCL), said Corin.




Let's personalize your content