Triastek Receives FDA IND Clearance for 3D Printed Medicine for the Treatment of Ulcerative Colitis
Additive Manufacturing
NOVEMBER 30, 2022
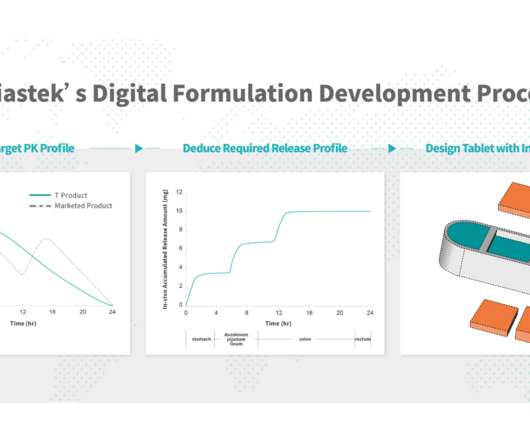
21, 2022 — Triastek, Inc. , 21, 2022 — Triastek, Inc. , With a unique 3D dosage form design, T21 can reach the targeted colon segment of the GI tract, thus permitting use of a lower drug dose than the reference listed drug, which provides systemic exposure. NANJING, China, Nov.














Let's personalize your content